W.B.C.S. Main 2018 Question Answer – Medical Science – Adverse Drug Reactions.
WBCS মেইনস ২০১৮ প্রশ্ন উত্তর – চিকিত্সা বিজ্ঞান – প্রতিকূল ড্রাগ প্রতিক্রিয়া।
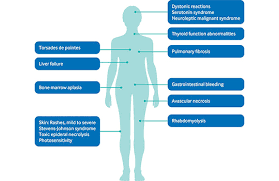
1)Write a note on Adverse Drug Reactions.
Adverse drug reactions can be considered a form of toxicity; however, toxicity is most commonly applied to effects of overingestion (accidental or intentional) or to elevated blood levels or enhanced drug effects that occur during appropriate use (eg, when drug metabolism is temporarily inhibited by a disorder or another drug). For information on toxicity of specific drugs see the table Symptoms and Treatment of Specific Poisons. Side effect is an imprecise term often used to refer to a drug’s unintended effects that occur within the therapeutic range.Continue Reading W.B.C.S. Main 2018 Question Answer – Medical Science – Adverse Drug Reactions.
Because all drugs have the potential for adverse drug reactions, risk-benefit analysis (analyzing the likelihood of benefit vs risk of ADRs) is necessary whenever a drug is prescribed.
In the US, 3 to 7% of all hospitalizations are due to adverse drug reactions. ADRs occur during 10 to 20% of hospitalizations; about 10 to 20% of these ADRs are severe. These statistics do not include the number of ADRs that occur in ambulatory and nursing home patients. Although the exact number of ADRs is not certain, ADRs represent a significant public health problem that is, for the most part, preventable .
Incidence and severity of adverse drug reactions vary by patient characteristics (eg, age, sex, ethnicity, coexisting disorders, genetic or geographic factors) and by drug factors (eg, type of drug, administration route, treatment duration, dosage, bioavailability). Incidence is higher with advanced age and polypharmacy. ADRs are more severe among the elderly (see Drug-Related Problems in the Elderly), although age per se may not be the primary cause. The contribution of prescribing and adherence errors to the incidence of ADRs is unclear.
Etiology
Most adverse drug reactions are dose-related; others are allergic or idiosyncratic. Dose-related ADRs are usually predictable; ADRs unrelated to dose are usually unpredictable.
Dose-related ADRs are particularly a concern when drugs have a narrow therapeutic index (eg, hemorrhage with oral anticoagulants). ADRs may result from decreased drug clearance in patients with impaired renal or hepatic function or from drug-drug interactions.
Allergic ADRs are not dose-related and require prior exposure. Allergies develop when a drug acts as an antigen or allergen. After a patient is sensitized, subsequent exposure to the drug produces one of several different types of allergic reaction. Clinical history and appropriate skin tests can sometimes help predict allergic ADRs.
Idiosyncratic ADRs are unexpected ADRs that are not dose-related or allergic. They occur in a small percentage of patients given a drug. Idiosyncrasy is an imprecise term that has been defined as a genetically determined abnormal response to a drug, but not all idiosyncratic reactions have a pharmacogenetic cause. The term may become obsolete as specific mechanisms of ADRs become known.
Symptoms and Signs
Adverse drug reactions are usually classified as mild, moderate, severe, or lethal (see table Classification of Adverse Drug Reactions [ADRs]). Severe or lethal ADRs may be specifically mentioned in black box warnings in the physician prescribing information provided by the manufacturer.
Symptoms and signs may manifest soon after the first dose or only after chronic use. They may obviously result from drug use or be too subtle to identify as drug-related. In the elderly, subtle ADRs can cause functional deterioration, changes in mental status, failure to thrive, loss of appetite, confusion, and depression.
Diagnosis
Symptoms that occur soon after a drug is taken are often easily connected with use of a drug. However, diagnosing symptoms due to chronic drug use requires a significant level of suspicion and is often complicated. Stopping a drug is sometimes necessary but is difficult if the drug is essential and does not have an acceptable substitute. When proof of the relationship between drug and symptoms is important, rechallenge should be considered, except in the case of serious allergic reactions.
The incidence of severe or fatal adverse drug reactions is very low (typically < 1 in 1000) and may not be apparent during clinical trials, which are typically not powered to detect low-incidence ADRs. Thus, these ADRs may not be detected until after a drug is released to the general public and is in widespread use. Clinicians should not assume that because a drug is on the market that all ADRs are known. Postmarketing surveillance is extremely important for tracking low-incidence ADRs.
Our own publications are available at our webstore (click here).
For Guidance of WBCS (Exe.) Etc. Preliminary , Main Exam and Interview, Study Mat, Mock Test, Guided by WBCS Gr A Officers , Online and Classroom, Call 9674493673, or mail us at – mailus@wbcsmadeeasy.in
Visit our you tube channel WBCSMadeEasy™ You tube Channel
Please subscribe here to get all future updates on this post/page/category/website



 Toll Free 1800 572 9282
Toll Free 1800 572 9282  mailus@wbcsmadeeasy.in
mailus@wbcsmadeeasy.in