Phenols And Ethers – Chemistry Notes – For W.B.C.S. Examination.
ফেনোল এবং ইথার – রসায়ন নোট – WBCS পরীক্ষা।
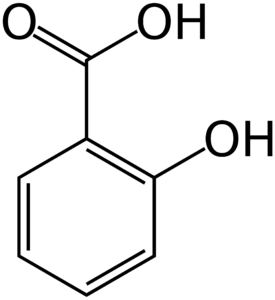
Phenols:
Continue Reading Phenols And Ethers – Chemistry Notes – For W.B.C.S. Examination.
Preparation:


Physical Properties of Phenols
-
Phenol is a colorless, toxic, corrosive, needle shaped solid.
-
Phenol soon liquifies due to high hygroscopic nature.
-
Phenol is less soluble in water, but readily soluble in organic solvents.
-
Simplest phenols, because of hydrogen bonding have quite high boiling points.
-
o-nitrophenol is, steam volatile and also is less soluble in water because of intramolecular hydrogen bonding
Chemical Properties of Phenols
a) Formation of Esters
Phenyl esters (RCOOAr) are not formed directly from RCOOH. Instead, acid chlorides or anhydrides are reacted with ArOH in the presence of strong base
(CH3CO)2O + C6H5OH + NaOH → CH3COOC6H5 + CH3COONa + H2O
Phenyl acetate
C6H5COCl + C6H5OH + NaOH → C6H5COOC6H5 + Na+Cl– + H2O
Phenyl benzoate

e) Electrophilic SubstitutionThe —OH and even more so the —O(phenoxide) are strongly activating ortho ,para – directing

Special mild conditions are needed to achieve electrophilic monosubstituion in phenols because their high reactivity favors both polysubstitution and oxidation




Ethers:
Physical Properties of Ethers
-
Physical state, colour and odour: Dimethyl ether and ethyl methyl ether is gas at ordinary temperature while the other lower homologues of ethers are colourless liquid with characteristic ‘ether smell’.
-
Dipole nature: Ethers have a tetrahedral geometry i.e., oxygen is sp3 hybridized. The C— O—C angle in ethers is 110°. Because of the greater electronegativity of oxygen than carbon, the C—O bonds are slightly polar and are inclined to each other at an angle of 110°, resulting in a net dipole moment.

Bond angle of ether is greater than that of tetrahedral bond angle of 109°28′. -
Solubility and boiling point: Due to the formation of less degree of hydrogen bonding, ethers have lower boiling point than their corresponding isomeric alcohols and are slightly soluble in water.
Preparation of Ethers:
a) From alcohols:

Order of dehydration of alcohol leading to formation of ethers: 1° > 2° > 3°

b) Williamson’s synthesis:
R-X + Na+ –O-R’ → R-O-R’ + Na+ X–
In case of tertiary substrate elimination occurs giving alkenes.
From alkenes:.

From Grignard reagent: Treating a – halo ethers with suitable Grignard reagents.

On standing in contact with air, most aliphatic ethers are converted slowly into unstable peroxides. The presence of peroxides is indicated by formation of a red colour when the ether is shaken with an aqueous solution of ferrous ammonium sulfate and potassium thiocyanate
? 
Please subscribe here to get all future updates on this post/page/category/website


 Toll Free 1800 572 9282
Toll Free 1800 572 9282  mailus@wbcsmadeeasy.in
mailus@wbcsmadeeasy.in