W.B.C.S. Examination Notes On – Joule Thomson Effect – Chemistry.
Chemistry is one such optional offered in the W.B.C.S. Examination. It is one of the optional subjects that is preferred by the aspirants graduated with the subject. Chemistry optional is a scoring subject if aspirants have sound knowledge in it. Similar to other optional papers, chemistry also has two papers. The paper I of chemistry optional deals with Inorganic Chemistry and Physical Chemistry. Paper-II completely deals with Organic Chemistry.Following previous years question papers are a must when it comes to optional subjects.For several years, James Prescott Joule and William Thomson – both British physicists – worked in collaboration, conducting experiments designed to analyze and advance thermodynamics. In 1852, the researchers made a particularly notable discovery.Continue Reading W.B.C.S. Examination Notes On – Joule Thomson Effect – Chemistry.
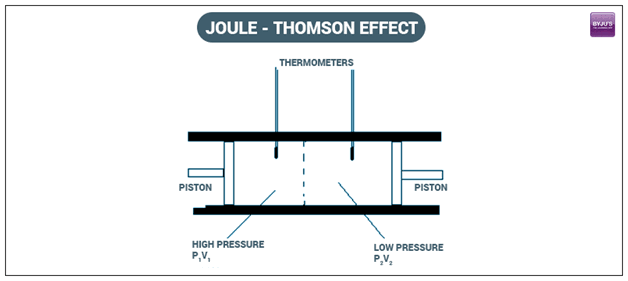
They found that a temperature change can occur in a gas as a result of a sudden pressure change over a valve. Known as the Joule-Thomson effect (or sometimes the Thomson-Joule effect), this phenomenon has proven to be important in the advancement of refrigeration systems as well as liquefiers, air conditioners, and heat pumps. It is also the effect that is responsible for a tire valve getting cold when you let out the air from a bicycle tire.
The temperature change pertaining to the Joule-Thomson effect can occur when a flowing gas passes through a pressure regulator, which acts as a throttling device, valve, or porous plug. Here, a temperature change is not necessarily desirable. To balance out any Joule-Thomson related temperature changes, a heating or cooling element can be used.
This is an adiabatic throttling process. No heat or mechanical work is exchanged with the environment. Fundamental thermodynamic definitions can be used to develop an energy balance for the flow process into and out of the porous section, with 1 representing the inlet and 2 representing the outlet.
where is the enthalpy and is the velocity (m/s). Here, any magnetic, electric, and nuclear energy contributions are neglected. For gas flows at moderate velocities, it is safe to disregard the kinetic energy change in comparison to any enthalpy changes.
Therefore, it is evident that the process happens at constant enthalpy – in other words, it is isenthalpic. Most engineers remember from their textbooks that an enthalpy change can be calculated from the material property heat capacity.
At this point, from the equation above, one might jump to the conclusion that if is 0, then must also be 0, assuming that is never 0. Such a conclusion contradicts the experimental findings from Thomson and Joule. The two physicists found that some gases actually change in temperature at throttling. But how can this be explained? The answer lies in some thermodynamic reasoning and the concept of ideal versus real gases. Unfortunately, is not entirely true; it is a special case for ideal gases (and liquids).Also Read ,W.B.C.S. Examination Notes On – Sociology – Demonstration Of Sociological Proof.
Looking at a more general situation, is a thermodynamic state function. According to the so-called Gibbs’ phase rule, the function must have two degrees of freedom for a substance with a fixed composition in one phase. This means that the state of a gas can be exactly determined, provided that the values of exactly two other state functions are known. Determining the enthalpy can be accomplished by determining two other arbitrary state functions. The options include: temperature (), pressure (), entropy (), specific volume (), or internal energy () and more. The only requirement is that two of them are determined.
Here’s an example that uses temperature and pressure:
A small change, , in the enthalpy will, by the chain rule, be:
The indication represents a partial derivative of with respect to , where is the second degree of freedom selected and is held constant. This can be integrated and replaced with the definition of
The first term on the right-hand side is the enthalpy change of an ideal gas, and the second term is the additional contribution due to the nonideality of the gas. This can be interpreted as the work that must be exerted to overcome intermolecular forces. An ideal gas, by definition, has no intermolecular forces. For an isenthalpic process, Eq. also helps in the interpretation of any slight temperature change, as it is able to provide the exact amount of thermal energy conversion needed to overcome intermolecular forces.
Revisiting the experiments of Thomson and Joule, the two men found it practical to relate their observations of temperature change at constant enthalpy to something measurable: How much does the temperature change for a small change in pressure, holding the enthalpy fixed? They referred to it as the Joule-Thomson coefficient.
Please subscribe here to get all future updates on this post/page/category/website


 +919674493673
+919674493673  mailus@wbcsmadeeasy.in
mailus@wbcsmadeeasy.in