Atomic Radii – Chemistry Notes – For W.B.C.S. Examination.
পারমাণবিক ব্যাসার্ধ – রসায়ন নোট – WBCS পরীক্ষা।
Atomic radii is useful for determining many aspects of chemistry such as various physical and chemical properties. The periodic table greatly assists in determining atomic radius and presents a number of trends.Continue Reading Atomic Radii – Chemistry Notes – For W.B.C.S. Examination.
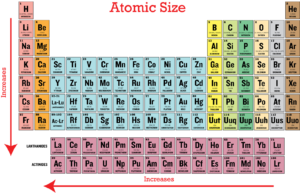
Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost orbital of electron. In simpler terms, it can be defined as something similar to the radius of a circle, where the center of the circle is the nucleus and the outer edge of the circle is the outermost orbital of electron. As you begin to move across or down the periodic table, trends emerge that help explain how atomic radii change.
The effective nuclear charge (ZeffZeff) of an atom is the net positive charge felt by the valence electron. Some positive charge is shielded by the core electrons therefore the total positive charge is not felt by the valence electron. A detailed description of shielding and effective nuclear charge can be found here. ZeffZeff greatly affects the atomic size of an atom. So as the ZeffZeff decreases, the atomic radius will grow as a result because there is more screening of the electrons from the nucleus, which decreases the attraction between the nucleus and the electron. Since ZeffZeffdecreases going down a group and right to left across the periodic table, the atomic radius will increase going down a group and right to left across the periodic table.
Types of Radius with Respect to Types of Bonds
Determining the atomic radii is rather difficult because there is an uncertainty in the position of the outermost electron – we do not know exactly where the electron is. This phenomenon can be explained by the Heisenberg Uncertainty Principle. To get a precise measurement of the radius, but still not an entirely correct measurement, we determine the radius based on the distance between the nuclei of two bonded atoms. The radii of atoms are therefore determined by the bonds they form. An atom will have different radii depending on the bond it forms; so there is no fixed radius of an atom.
Covalent Radius
When a covalent bond is present between two atoms, the covalent radius can be determined. When two atoms of the same element are covalently bonded, the radius of each atom will be half the distance between the two nuclei because they equally attract the electrons. The distance between two nuclei will give the diameter of an atom, but you want the radius which is half the diameter.
Ionic Radius
The ionic radius is the radius of an atom forming ionic bond or an ion. The radius of each atom in an ionic bond will be different than that in a covalent bond. This is an important concept. The reason for the variability in radius is due to the fact that the atoms in an ionic bond are of greatly different size. One of the atoms is a cation, which is smaller in size, and the other atom is an anion which is a lot larger in size. So in order to account for this difference, one most get the total distance between the two nuclei and divide the distance according to atomic size. The bigger the atomic size, the larger radius it will have. This is depicted in Figure 2 as shown below where the cation is displayed on the left as X+, and clearly has a smaller radius than the anion, which is depicted as Y- on the right.
Please subscribe here to get all future updates on this post/page/category/website


 +919674493673
+919674493673  mailus@wbcsmadeeasy.in
mailus@wbcsmadeeasy.in