Chemistry Notes On – Ionization Energies – For W.B.C.S. Examination.
রসায়ন ণোট – আয়নায়ন শক্তি – WBCS পরীক্ষা।
Ionization energy is the energy required to remove an electron from a gaseous atom or ion. The first or initial ionization energy or Ei of an atom or molecule is the energy required to remove one mole of electrons from one mole of isolated gaseous atoms or ions.Ionization energy is in indicator of reactivity. Ionization energy is important because it can be used to help predict the strength of chemical bonds.Continue Reading Chemistry Notes On – Ionization Energies – For W.B.C.S. Examination.
Ionization Energy Trend in the Periodic Table
Ionization, together with atomic and ionic radius, electronegativity, electron affinity, and metallicity, follows a trend on the periodic table of elements.
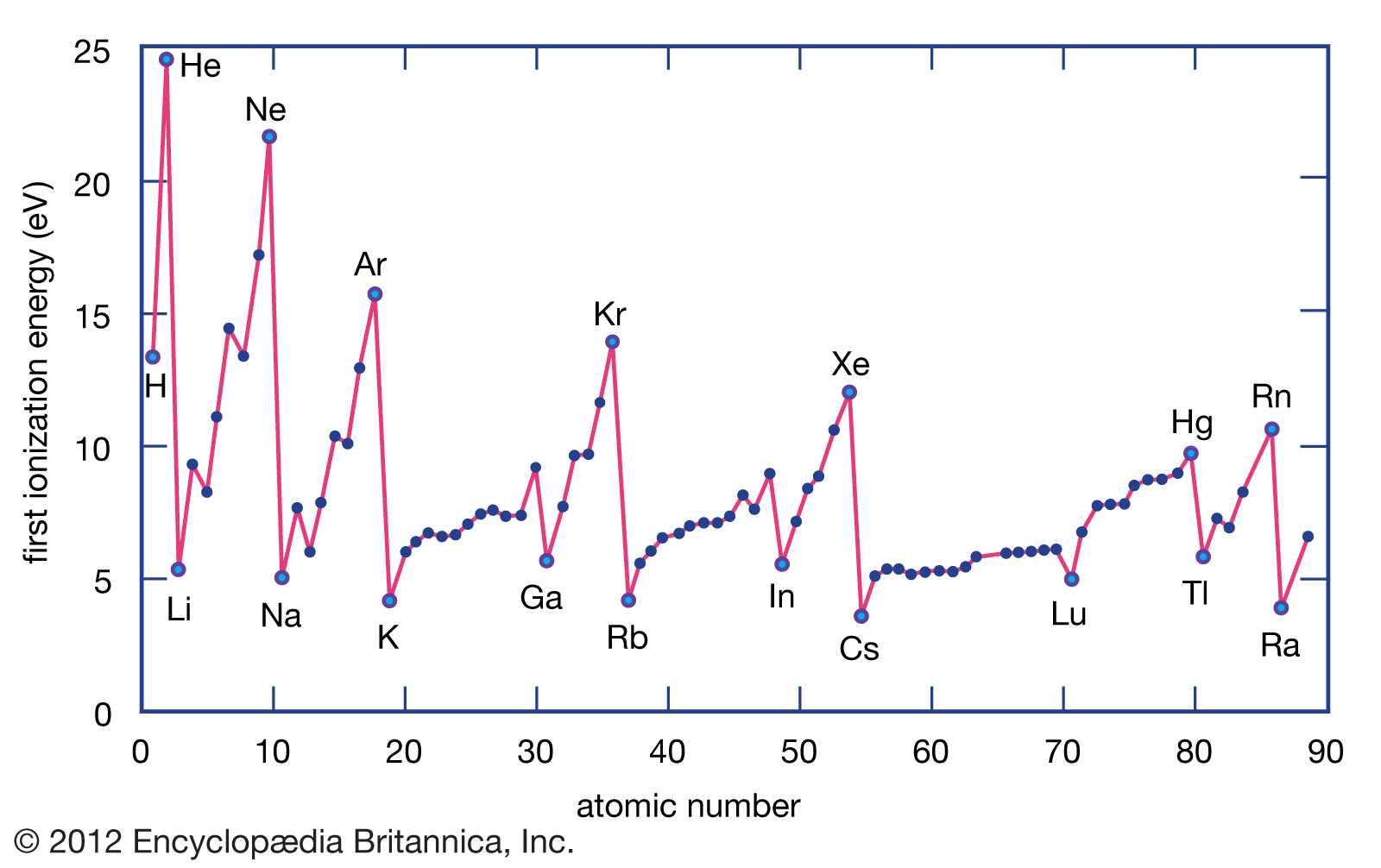
- Ionization energy generally increases moving from left to right across an element period (row). This is because the atomic radius generally decreases moving across a period, so there is a greater effective attraction between the negatively charged electrons and positively-charged nucleus. Ionization is at its minimum value for the alkali metal on the left side of the table and a maximum for the noble gas on the far right side of a period. The noble gas has a filled valence shell, so it resists electron removal.
- Ionization decreases moving top to bottom down an element group (column). This is because the principal quantum number of the outermost electron increases moving down a group. There are more protons in atoms moving down a group (greater positive charge), yet the effect is to pull in the electron shells, making them smaller and screening outer electrons from the attractive force of the nucleus. More electron shells are added moving down a group, so the outermost electron becomes increasingly distance from the nucleus.
First, Second, and Subsequent Ionization Energies
The energy required to remove the outermost valence electron from a neutral atom is the first ionization energy. The second ionization energy is that required to remove the next electron, and so on. The second ionization energy is always higher than the first ionization energy. Take, for example, an alkali metal atom. Removing the first electron is relatively easy because its loss gives the atom a stable electron shell. Removing the second electron involves a new electron shell that is closer and more tightly bound to the atomic nucleus.
The first ionization energy of hydrogen may be represented by the following equation:
H(g) → H+(g) + e–
ΔH° = -1312.0 kJ/mol
Exceptions to the Ionization Energy Trend
If you look at a chart of first ionization energies, two exceptions to the trend are readily apparent. The first ionization energy of boron is less than that of beryllium and the first ionization energy of oxygen is less than that of nitrogen.
The reason for the discrepancy is due to the electron configuration of these elements and Hund’s rule. For beryllium, the first ionization potential electron comes from the 2s orbital, although ionization of boron involves a 2p electron. For both nitrogen and oxygen, the electron comes from the 2p orbital, but the spin is the same for all 2p nitrogen electrons, while there is a set of paired electrons in one of the 2p oxygen orbitals.
Our own publications are available at our webstore (click here).
For Guidance of WBCS (Exe.) Etc. Preliminary , Main Exam and Interview, Study Mat, Mock Test, Guided by WBCS Gr A Officers , Online and Classroom, Call 9674493673, or mail us at – mailus@wbcsmadeeasy.in
Visit our you tube channel WBCSMadeEasy™ You tube Channel
Please subscribe here to get all future updates on this post/page/category/website


 +919674493673
+919674493673  mailus@wbcsmadeeasy.in
mailus@wbcsmadeeasy.in