Radius Ratio Rule – Physics Notes – For W.B.C.S. Examination.
ব্যাসার্ধের অনুপাতের বিধি – পদার্থবিজ্ঞানের নোট – WBCS পরীক্ষা।
Physics is an optional subject for W.B.C.S. Mains Exam. It is a discipline of science which deals with properties and nature of matter and energy. It includes light, heat, mechanics and other related themes. Just go through the W.B.C.S. Main optional Physics syllabus and you will find that aspirants those who are from Physics and Electrical engineering background can handle this optional subject. W.B.C.S. aspirants coming from non-electronics background must put extra hard work and endeavor to ace this optional. Ionic Crystals comprises many cations and anions. We know that anions are larger in size and surround the smaller cations. They are arranged in space such that anions and cations touch each other and produce maximum stability.Continue Reading Radius Ratio Rule – Physics Notes – For W.B.C.S. Examination.
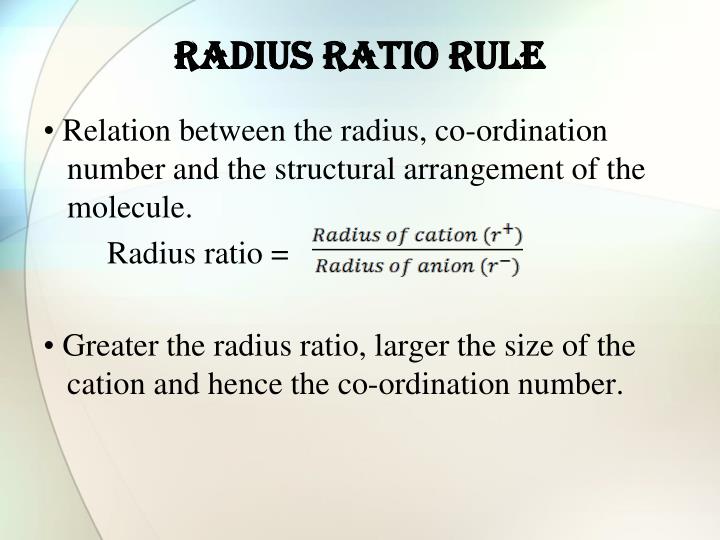
This stability of the ionic crystals can be explained on the basis of radius ratio. Therefore, radius ratio is the ratio of cation to the ratio of an anion. Here, Ratio of cation= r, Ratio of anion = R. Thus, Radius ratio = (r/R). Limiting radius ratio helps in expressing the range of radius ratio.
Definition of the Radius Ratio Rule
Radius Ratio refers to as the ration of smaller ionic radius (cation) by the ratio of larger ionic radius (anion). Hence, Radius ration ρ = rs/rl.
Importance of the Radius Ratio Rule
This rule helps in the determination of arrangement of ions in various types of crystal structures. It also helps to determine the stability of an ionic crystal structure. For instance, larger cations will fill the larger voids like cubic sites whereas smaller cations will fill the smaller voids such as tetrahedral sites.
It is also possible to predict the coordination number of any compound. Hence, the radius ratio rule helps in determining the structure of ionic solids.Also Read , Essay Composition On – Humanism – For W.B.C.S. Examination.
Examples of Radius Ratio Rule
The ratio of radii of the ions can affect the arrangement of ions in a crystal. Moreover, the limiting ratio has to be greater than 0.414, (radius ratio greater than 0.414) to fit an octahedral arrangement of anions. In this formation, cations will be able to accommodate 6 anions.
However, radius ration in between 0.225 to 0.414 will be able to fit into tetrahedral voids in the crystal lattice thereby preferring tetrahedral coordination and above 0.414 will prefer octahedral coordination. For example, if we consider an ion zinc sulfide, the radius ration will be
![]()
Therefore, zinc ion will favour tetrahedral voids in the closely arranged lattice of sulfide ions. However, in case of larger cations such as caesium, the radius ratio is larger than the limit of the coordination number of 6. Hence, the caesium ions will fit cubic sites so the coordination number will increase to 8 in the chloride ions lattice.
![]()
The electrostatic interaction between charged spheres is responsible for the formation of bonding in an ionic model. The determination of the sizes of the ionic radius is possible by the internuclear separation of the separate contributions of the anion from cation.
The radius of one ion is calculated on the basis of a standard ion (by assuming the value of one ion). The standard ion is oxide ion which helps in the determination of the other ions. This is because an oxide ion occurs in combination with many different elements. Moreover, an oxide ion is comparatively unpolarizable. Therefore, the significant change in the size is negligible on the basis of the counterion present.
Ionic radius is helpful in the prediction of crystal structures including lengths of the axes, lattice parameters, etc. However, this prediction is possible provided the values of the radius of the ions are taken from the same origin or same reference ion. This is important for achieving the correct relative sizes.
It is essential to understand that ionic radius differs on the basis of coordination number. The increase in coordination number results in the ions moving further away from the central ion to fit more ions. Therefore, increase in coordination number will increase the interionic separation and decrease the short ranged repulsion. This, in turn, will allow the electrons present on the central ion to expand thereby increasing the size of the central ion.
Please subscribe here to get all future updates on this post/page/category/website


 +919674493673
+919674493673  mailus@wbcsmadeeasy.in
mailus@wbcsmadeeasy.in