W.B.C.S. Examination Notes On – Chemistry – Clausius-Clapeyron Equation.
WBCS পরীক্ষার নোট – রসায়ন – ক্লাসিয়াস-ক্ল্যাপিয়রন সমীকরণ।
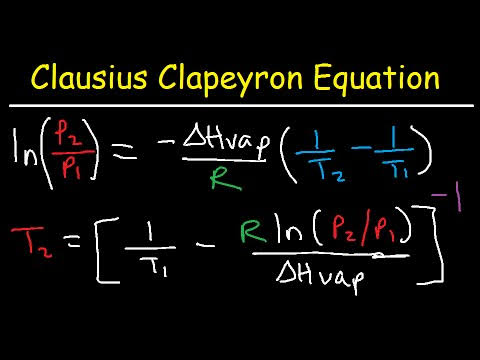
Chemistry is one such optional offered in the W.B.C.S. Examination. It is one of the optional subjects that is preferred by the aspirants graduated with the subject. Chemistry optional is a scoring subject if aspirants have sound knowledge in it. Similar to other optional papers, chemistry also has two papers. The paper I of chemistry optional deals with Inorganic Chemistry and Physical Chemistry. Paper-II completely deals with Organic Chemistry.Following previous years question papers are a must when it comes to optional subjects.The relationship between the temperature of a liquid and its vapor pressure is not a straight line. The vapor pressure of water, for example, increases significantly more rapidly than the temperature of the system. This behavior can be explained with the Clausius-Clapeyron equation.Continue Reading W.B.C.S. Examination Notes On – Chemistry – Clausius-Clapeyron Equation.
 |
According to this equation, the rate at which the natural logarithm of the vapor pressure of a liquid changes with temperature is determined by the molar enthalpy of vaporization of the liquid, the ideal gas constant, and the temperature of the system. If we assume that Hvap does not depend on the temperature of the system, the Clausius-Clapeyron equation can be written in the following integrated form where C is a constant.Chemistry optional syllabus is available here, CHEMISTRY OPTIONAL SYLLABUS FOR W.B.C.S. Examination.
 |
This form of the Clausius-Clapeyron equation has been used to measure the enthalpy of vaporization of a liquid from plots of the natural log of its vapor pressure versus temperature. For our purposes, it would be more useful to take advantage of logarithmic mathematics to write this equation as follows.
 |
Because the molar enthalpy of vaporization of a liquid is always a positive number, this equation suggests that the logarithm of the vapor pressure will increase as the temperature of the system increases. Since the vapor pressure of the liquid increases much more rapidly than its natural logarithm, we get the behavior observed in the plot of vapor pressure versus temperature.To view, Notes On Chemistry-Hydrogen Economy-Hard Water types & treatment-Hydrogen Peroxide,-Acid-Base-Salt-For WBCS Main Exam , Click on the above link.
The derivation will be given for a liquid-vapor equilibrium interface but it equally well applies to the interface between any two phases.
Let sv and sl be the specific entropy of the vapor and liquid phases, respectively. The pressure and temperature of the two phases are equal. The chemical potential μ is equal on either side of the phase boundary curve. Therefore the changes dμ in the chemical potential for movements along the phase boundary curve are also equal. This means that
dμv = Vvdp – svdT = Vldp – sldT = dμl
Solving for dp gives,
dp = (sv – sl)dT/(Vv – Vl)
or, equivalently
dp/dT = (sv – sl)/(Vv – Vl)
But the change in entropy Δs for the phase change is just L/T so
dp/dT = L/(T(Vv – Vl))
This is the Clausius-Clapeyron equation.
Please subscribe here to get all future updates on this post/page/category/website


 +919674493673
+919674493673  mailus@wbcsmadeeasy.in
mailus@wbcsmadeeasy.in