W.B.C.S. Examination Notes On – Weak Acid – Chemistry Notes.
A weak acid is an acid that partially dissociates into its ions in an aqueous solution or water. In contrast, a strong acid fully dissociates into its ions in water. The conjugate base of a weak acid is a weak base, while the conjugate acid of a weak base is a weak acid. At the same concentration, weak acids have a higher pH value than strong acids.Continue Reading W.B.C.S. Examination Notes On – Weak Acid – Chemistry Notes.
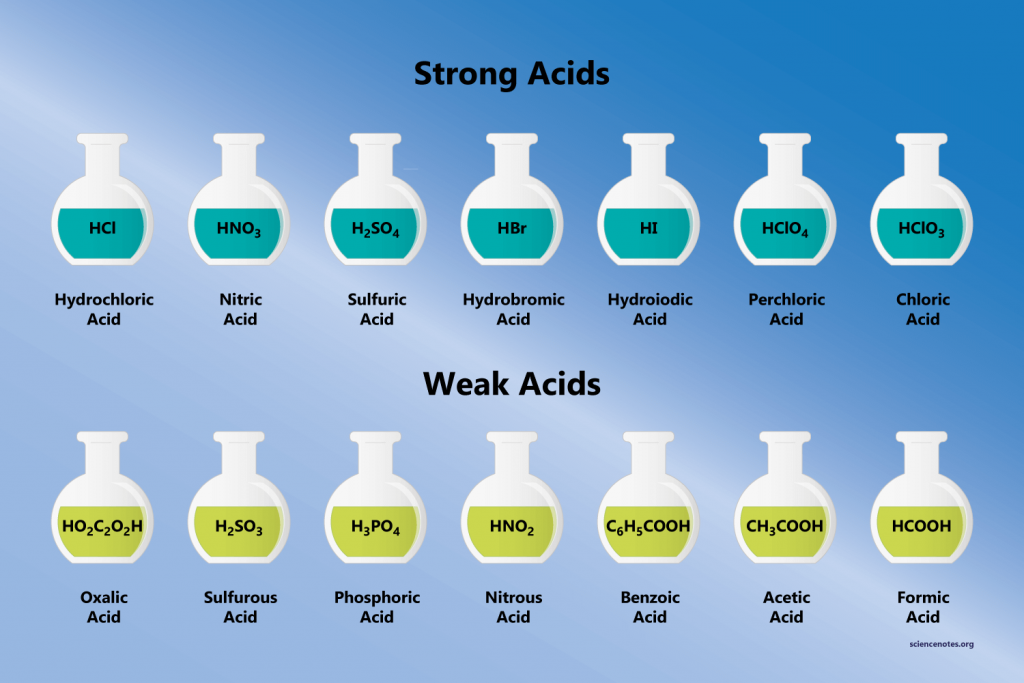
Weak acids are much more common than strong acids. They are found in daily life in vinegar (acetic acid) and lemon juice (citric acid), for example.
| Common Weak Acids | |
|---|---|
| Acid | Formula |
| acetic acid (ethanoic acid) | CH3COOH |
| formic acid | HCOOH |
| hydrocyanic acid | HCN |
| hydrofluoric acid | HF |
| hydrogen sulfide | H2S |
| trichloracetic acid | CCl3COOH |
| water (both weak acid and weak base) | H2O |
Ionization of Weak Acids
The reaction symbol for a strong acid ionizing in water is a simple arrow facing from left to right. On the other hand, the reaction arrow for a weak acid ionizing in water is a double arrow, indicating that both the forward and reverse reactions occur at equilibrium. At equilibrium, the weak acid, its conjugate base, and the hydrogen ion are all present in the aqueous solution. The general form of the ionization reaction is:
HA ⇌ H++A−
For example, for acetic acid, the chemical reaction takes the form:
H3COOH ⇌ CH3COO– + H+
The acetate ion (on the right or product side) is the conjugate base of acetic acid.
Why Are Weak Acids Weak?
Whether or not an acid completely ionizes in water depends on the polarity or distribution of the electrons in a chemical bond. When two atoms in a bond have nearly the same electronegativity values, the electrons are evenly shared and spend equal amounts of time associated with either atom (a nonpolar bond). On the other hand, when there is a significant electronegativity difference between the atoms, there is a separation of charge; as a result, electrons are drawn more to one atom than to the other (polar bond or ionic bond).
Hydrogen atoms have a slight positive charge when bonded to an electronegative element. If there is less electron density associated with hydrogen, it becomes easier to ionize and the molecule becomes more acidic. Weak acids form when there isn’t enough polarity between the hydrogen atom and the other atom in the bond to allow for easy removal of the hydrogen ion.
Another factor that affects the strength of an acid is the size of the atom bonded to hydrogen. As the size of the atom increases, the strength of the bond between the two atoms decreases. This makes it easier to break the bond to release the hydrogen and increases the strength of the acid.
Please subscribe here to get all future updates on this post/page/category/website


 +919674493673
+919674493673  mailus@wbcsmadeeasy.in
mailus@wbcsmadeeasy.in